କାବୋଜାଣ୍ଟିନିବ
 | |
| Clinical data | |
|---|---|
| Pronunciation | ka" boe zan' ti nib[୨] |
| Trade names | Cometriq, Cabometyx, others |
| Synonyms | XL184, BMS907351 |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a613015 |
| data |
|
| Pregnancy category | |
| Routes of administration | By mouth |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | ≥99.7% |
| Metabolism | Liver (CYP3A4-mediated) |
| Elimination half-life | 110 hours |
| Excretion | Feces (54%), urine (27%) |
| Identifiers | |
| |
| ECHA InfoCard | 100.221.147 |
| Chemical and physical data | |
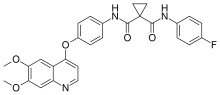
| Formula | C28H24FN3O5 |
| Molar mass | ୫୦୧.୫୧ g·mol−1 |
| 3D model (JSmol) | |
| |
| |
କାବୋଜାଣ୍ଟିନିବ, ଅନ୍ୟମାନଙ୍କ ମଧ୍ୟରେ ବିକ୍ରୟ ନାମ କୋମେଟ୍ରିକ (Cometriq) ଏବଂ କାବୋମରଟିକ୍ସ (Cabometyx), ଏକ ଔଷଧ ଯାହା ମେଡୁଲାରୀ ଥାଇରଏଡ କର୍କଟ, ବୃକ୍କ କୋଷ କର୍କଟ ଏବଂ ହେପାଟୋସେଲୁଲାର୍ କର୍କଟ ରୋଗମାନଙ୍କର ଚିକିତ୍ସା ପାଇଁ ବ୍ୟବହୃତ ହୁଏ । [୯] [୧୦] ଏହା ଉନ୍ନତ ରୋଗ କିମ୍ବା ଅନ୍ୟାନ୍ୟ ଚିକିତ୍ସାରେ ବିଫଳ ହୋଇଥିବା କ୍ଷେତ୍ରରେ ବ୍ୟବହୃତ ହୁଏ । [୧୧] ଏହା ପାଟିରେ ଦିଆଯାଏ | [୧୧]
ସାଧାରଣ ପାର୍ଶ୍ୱ ପ୍ରତିକ୍ରିୟାରେ ତରଳ ଝାଡ଼ା, ଥକାପଣ, ଉଚ୍ଚ ରକ୍ତଚାପ, ବାନ୍ତି, ଏବଂ ଓଜନ ହ୍ରାସ ହୁଏ । [୧୦] ଅନ୍ୟାନ୍ୟ ପାର୍ଶ୍ୱ ପ୍ରତିକ୍ରିୟାରେ ରକ୍ତସ୍ରାବ, ଫିଷ୍ଟୁଲା, ରକ୍ତ ଜମାଟ ବାନ୍ଧିବା, ଯକୃତ ସମସ୍ୟା ଏବଂ ରିଭର୍ସିବଲ୍ ପୋଷ୍ଟୋରିଅର୍ ଲ୍ୟୁକୋଏନସେଫାଲୋପାଥି ସିଣ୍ଡ୍ରୋମ ଅନ୍ତର୍ଭୁକ୍ତ ହୋଇପାରେ। [୧୧] ଗର୍ଭାବସ୍ଥାରେ ବ୍ୟବହାର ଶିଶୁର କ୍ଷତି କରିପାରେ । [୧୧] ଏହା ଏମଇଟ, (MET), ଭିଇଜିଏଫଆର (VEGFR), ଏବଂ ଏଏକ୍ସଏଲ (AXL)ମାନଙ୍କର ଟାଇରୋସିନ୍ କାଇନେଜ ଇନହିବିଟର । [୧୧]
୨୦୧୨ରେ ଯୁକ୍ତରାଷ୍ଟ୍ରରେ ଏବଂ ୨୦୧୪ରେ ୟୁରୋପରେ ଚିକିତ୍ସା ବ୍ୟବହାର ପାଇଁ କାବୋଜାଣ୍ଟିନିବ ଅନୁମୋଦିତ ହୋଇଥିଲା [୯][୧୦] ୨୦୨୧ରେ ଯୁକ୍ତରାଜ୍ୟରେ ୬୦ମିଗ୍ରା ଔଷଧର ଏକ ମାସର ମୂଲ୍ୟ ୫୧୫୦ ପାଉଣ୍ଡ ଥିଲା । [୧୨] ଯୁକ୍ତରାଷ୍ଟ୍ରରେ ଏହି ରାଶିର ମୂଲ୍ୟ ପ୍ରାୟ ୨୨,୬୦୦ ଡଲାର ଅଟେ । [୧୩]
ଆଧାର
[ସମ୍ପାଦନା]- ↑ "Cabozantinib Use During Pregnancy". Drugs.com. 30 March 2020. Archived from the original on 3 December 2020. Retrieved 23 September 2020.
- ↑ "Cabozantinib". LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. 2012. Archived from the original on 11 January 2022. Retrieved 29 December 2021.
- ↑ "Cometriq EPAR". European Medicines Agency (EMA). Archived from the original on 2 October 2018. Retrieved 23 September 2020.
- ↑ "Cabometyx EPAR". European Medicines Agency (EMA). Archived from the original on 4 August 2020. Retrieved 23 September 2020.
- ↑ "Cabozantinib tablet (Cabometyx) UK Summary of Product Characteristics". UK Electronic Medicines Compendium. September 2016. Archived from the original on 2019-06-23. Retrieved 2021-10-05.
- ↑ "Cabozantinib capsule (Cometriq) UK Summary of Product Characteristics (SPC) - (eMC)". UK Electronic Medicines Compendium. November 2016. Archived from the original on 2019-06-23. Retrieved 2021-10-05.
- ↑ "Cabometyx- cabozantinib tablet". DailyMed. 21 July 2020. Archived from the original on 29 October 2020. Retrieved 23 September 2020.
- ↑ "Cometriq- cabozantinib kit Cometriq- cabozantinib capsule". DailyMed. 11 February 2020. Archived from the original on 29 October 2020. Retrieved 23 September 2020.
- ↑ ୯.୦ ୯.୧ "Cometriq EPAR". European Medicines Agency (EMA). Archived from the original on 2 October 2018. Retrieved 23 September 2020.
- ↑ ୧୦.୦ ୧୦.୧ ୧୦.୨ "DailyMed - CABOMETYX- cabozantinib tablet". dailymed.nlm.nih.gov. Archived from the original on 29 October 2020. Retrieved 29 December 2021.
- ↑ ୧୧.୦ ୧୧.୧ ୧୧.୨ ୧୧.୩ ୧୧.୪ "DailyMed - CABOMETYX- cabozantinib tablet". dailymed.nlm.nih.gov. Archived from the original on 29 October 2020. Retrieved 29 December 2021.
- ↑ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1017. ISBN 978-0857114105.
- ↑ "Cabometyx Prices, Coupons & Patient Assistance Programs". Drugs.com (in ଇଂରାଜୀ). Archived from the original on 4 March 2021. Retrieved 29 December 2021.
